I find the world of genetic engineering and the possibilities it enables fascinating. It sits right at the intersection of “technology with very high potential impact” and “transhumanist tech”.
I know that I want to build in this space, so I’ve been deep-diving to find the one niche to completely pour myself into. Here are some findings that sparked my curiosity!
Quick review of delivery/editing approaches
The main categories used in gene therapy are:
- Viral vectors (Viruses) – most commonly AAV (used in ~70% of trials) and lentivirus.
- Gene editing to correct in-situ – you might have heard about CRISPR; newer methods include “base editing” and “prime editing”
- Non-viral vectors – the buzziest example is lipid nano-particles (LNPs)
(Gene therapy = a vehicle that carries a genetic payload or performs a genetic modification)
Viral vectors (viruses!)
Viruses do an incredibly good job at sneaking in genes into our cells. In 1972 some smart cookies figured out that we could hijack their abilities by basically swapping their genetic contents for genes of our choice.
They’re the most commonly used method, but they have some key issues:
- Delivery – it’s hard to get them exactly where you want, in the quantity that you want (and nowhere else)
- Immunogenicity – they can trigger immune responses
- Manufacturing: incredibly expensive (estimates range from $2M to $3-7M per dose)
(on the other hand, consider that they can be a one-time cure!!) - Single-dose constraint: you cannot re-dose with the same virus because patients develop antibodies (+ sometimes they have preexisting ones!)
- Blood-brain barrier (BBB) – most can’t efficiently cross the BBB, meaning they can’t be used for brain indications. (NOTE: some teams are working on engineering capsids to fix this and improve targeting efficiency)
They (can) make permanent changes to the genome, which can be life-changing for patients with debilitating genetic diseases.
This also means that they open up possibilities for one-time cures. For example, ~90% of patients with severe blood disorders requiring frequent hospitalization and transfusions were able to live virtually transfusion-free after a single dose of Casgevy. In the case of Luxturna, patients with inherited blindness have maintained meaningful improvements in their vision 5 years after receiving treatment.
Something that caught my attention is that the first gene therapy trial was run in 1990, yet the first FDA approval didn’t happen until 2017 (less than 10 years ago!!). Behind this pause was an unfortunate fatal trial in 1999 (due to a severe immune response), which left the field on hold.
In-situ gene therapy
(I find these particularly fascinating)
CRISPR
The classic example is CRISPR-Cas9. The landmark paper on CRISPR editing was published in 2012 (little more than 10 years ago), the Nobel prize for this work was awarded in 2020, and the first in-human CRISPR-based therapy was approved in late 2023 (less than 2 years ago).
Did you hear that?? The first CRISPR-based therapy (Casgevy) was approved JUST TWO years ago!!!! GUYS!!
I’m not going to explain the mechanism behind CRISPR (there’s plenty of resources about this), but you should know that one of the main problems associated with it is that it relies on “cutting” (hence the name “molecular scissors”) DNA strands and forcing the cell to “glue” them back together (through its own repair mechanisms), and, unfortunately, while the cell does a great job at this most of the time, it sometimes leaves unwanted changes behind (usually deletions). This is great if your goal is to deactivate a certain gene, but not so great if you care about precision.
But CRISPR is not the state-of-the-art gene editing tool anymore! (although it is definitely the one in the most advanced stages of testing)
Base & Prime editing
The base editing method was first introduced in 2016. Base editing builds on CRISPR-Cas9 while avoiding the “cutting” mechanism to make hyper-targeted base-swaps through chemical means. Unfortunately it is limited to one single base pair change at a time** (only C→T, A→G), so it is best for single-nucleotide diseases.
The biggest difference with CRISPR is that it modifies bases * directly* (chemically), without having to rely on the cell’s repair mechanism to achieve the desired result.
(** Note: actually, it works in 4-8 nucleotide windows and edits all occurrences of the target nucleotide (eg A, T, C…) in that window at once. You can’t control which one. This is called “bystander editing”.)
But wait!!! We’re not done yet. The same lab that brought us base editing (David Liu’s at Harvard) kept cooking to bring us prime editing in 2019. Prime editing is AWESOME because it allows all sorts of desired modifications (it can change one or multiple nucleotides to whatever other nucleotides you want, and can make insertions or deletions of up to 30-50 nucleotides – all in a single edit), as it includes a “search-and-replace” feature where we can indicate precisely what sequence we want and where exactly (what to find, and what to put in its place). Isn’t this absolutely MIND-BLOWING?!?!
(The first human trial for prime editing started in 2024! Last year!!)
Yet base and prime editing are not going to replace CRISPR anytime soon. While they offer higher precision, they are much slower, more expensive and complex, and are best suited for cases needing exact single-letter corrections, or where unintended surrounding effects would be harmful. CRISPR-Cas9 remains the go-to method for simpler gene knock-outs or to delete large DNA regions.
NON-VIRAL METHODS
Non-viral systems are generally less immunogenic, easier to re-dose, and scalable, but often less efficient or less targeted than viral vectors.
LNPs
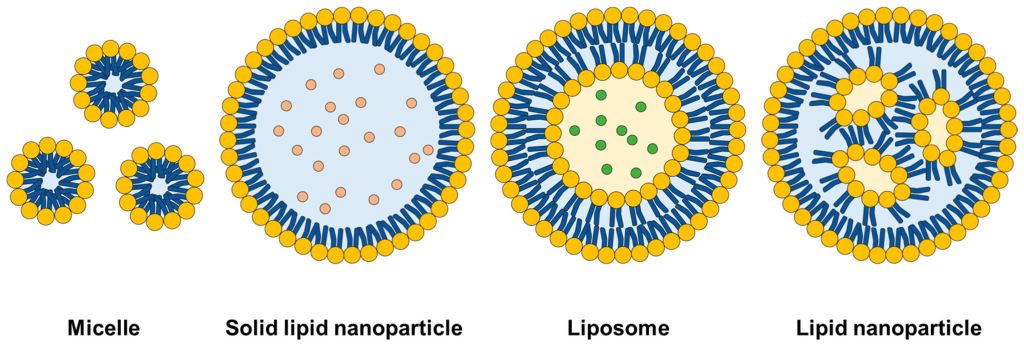
(I’ll briefly go through LNPs because they’re important, but I’ll leave these for the next mini-article)
LNPs are widely used for mRNA vaccines, and are being researched for gene therapies. (Pfizer’s and Moderna’s COVID vaccines used LNPs, while AstraZeneca, J&J and Sputnik V used adenoviral vectors)
Fun fact: LNPs were used in the first in-vivo CRISPR clinical trial to deliver the CRISPR system to the liver!
(Apparently, the first ever CRISPR trial apparently occurred in China in 2016, but it was ex-vivo – the patient’s cells were extracted and treated outside of the body)
(Note: the liver is the best target for LNPs as they naturally accumulate there after being injected intravenously, since the liver quickly filters nanoparticles circulating in the blood)
Focused ultrasound for BBB
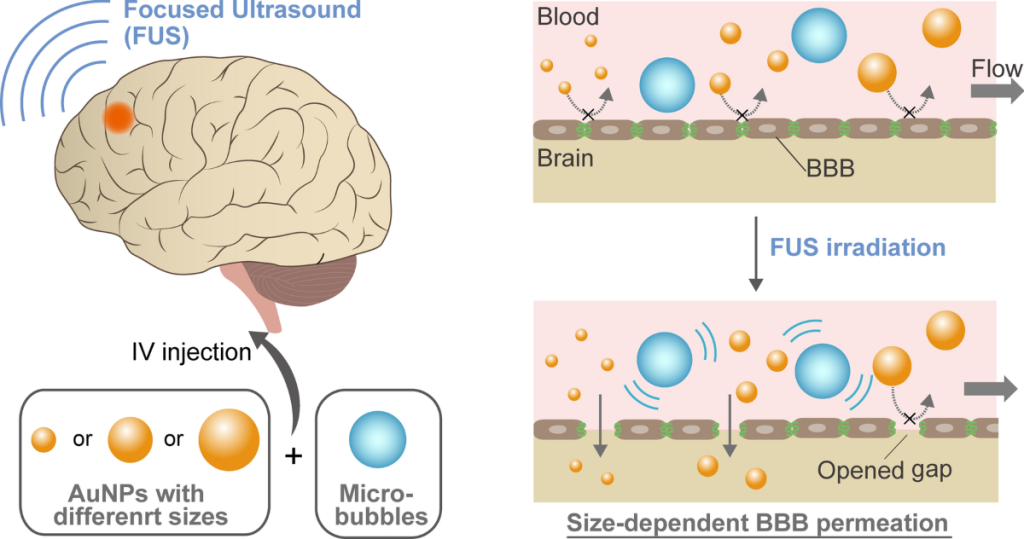
A bottleneck that really caught my attention is the blood-brain barrier (BBB). Some companies are working on engineering new types of AAV capsids that might have a shot at this, but one method to potentially deliver therapies to the brain that caught my eye is using focused ultrasound with microbubbles to temporarily open the BBB to let things through it.
This paper says “FUS with circulating microbubbles is currently the only method for inducing precise, transient, reversible, and noninvasive BBB opening (BBBO).”
This method has been proven safe in trials, but so far no trial has attempted to deliver genetic therapies through it.
Electroporation
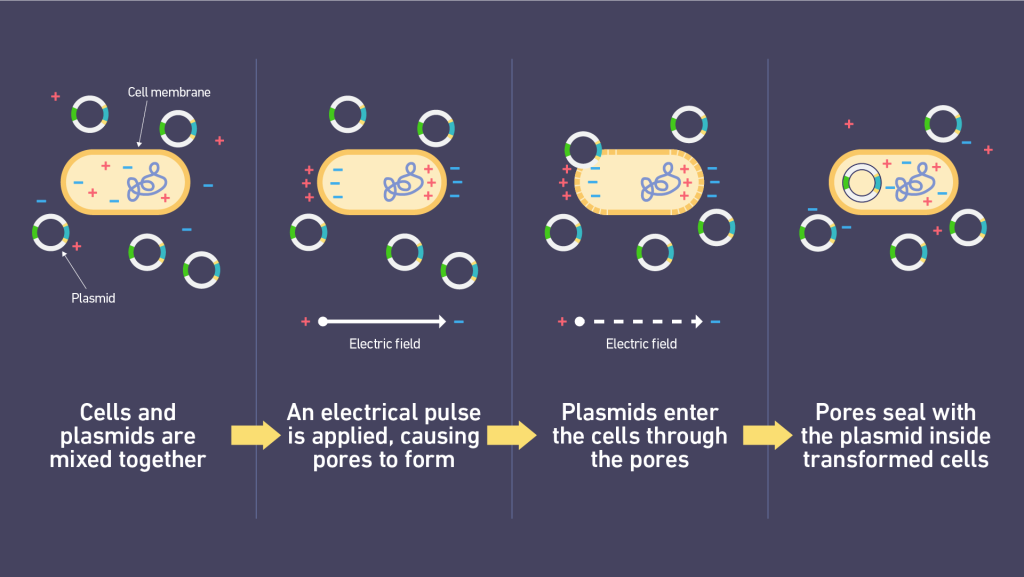
Another classic method of inserting things into biological materials is electroporation. Widely used in all sorts of processes in biology. The core idea is that you shoot pulses of electricity at cells to temporarily open up the pores on their membranes so that you can introduce DNA/RNA/proteins into them.
One of the standoud uses of electroporation is in the genetic engineering of T-Cells for CAR-T therapies, which have emerged as some of the most promising treatments for cancer to date.
It can deliver a wide variety of cargo (mRNA, RNPs, plasmid DNA, CRISPR), but can only be used ex-vivo.
(This is how you get plasmids into bacteria to genetically engineer them, BTW. You literally dump bacterial or mammalian cells plus whatever you want to get inside into a (special) liquid, “pew pew” them, and it’s done. Well, you do some prep first, but you get the idea.)
Topical gene therapies

I also learned that there are topical gene therapies??? Two notable ones are VYJUVEK (approved in 2023) and Zevaskyn (approved in 2025!).
Vyjuvek is basically a gel containing a modified version of the herpes virus that can’t replicate (so it doesn’t infect you and spread) but can efficiently deliver a working copy of a certain gene to skin cells. This was the first-ever topical gene therapy!
(It treats a genetic condition that causes the skin to be extremely prone to wounds because of a missing/defective gene, which is normally in charge of producing a protein Collagen VII that helps hold skin layers together).
(Can you imagine spreading a virus on your skin as a cream??)
In Zevaskyn, a biopsy is performed to extract the patient’s cells and treat them ex vivo (outside of the body) with a virus that inserts a working copy of a gene. These cells are grown into sheets, which are then grafted into the patient’s skin. (So you’re basically applying a sheet made of your own genetically-modified cells!)
Exosomes
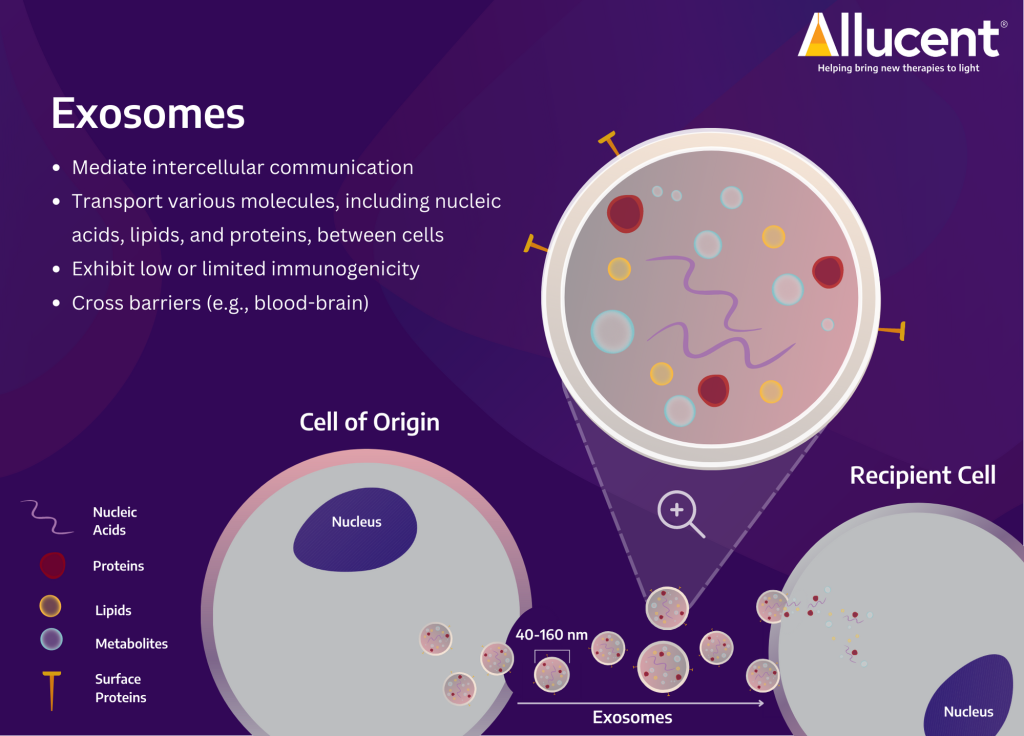
They are basically tiny (nano-sized) “bubbles” released by cells for intercellular communication. They occur naturally, and they’re involved in all sorts of biological processes, such as tissue repair and immune responses.
Exosomes can’t make gene edits directly, but they can be very helpful in transporting gene-editing tools (or other elements). They can be filled with mRNA, DNA, or proteins (including the CRISPR-Cas9 system). They’re ideal for transient treatments (days or weeks), and allow for repeated dosing (unlike viruses, since we develop antibodies for them).
Their main advantages are: their ability to penetrate barriers that are difficult for other methods (like the BBB), their target-specificity (avoid off-target effects by delivering to specific tissues), and their low immunogenicity.
The main downsides revolve around how quickly they get cleared from the body (low certainty for how much quantity of a therapy actually ends up in our target tissues), and how hard it is to manufacture them (for example, it’s currently not possible to manufacture homogeneous batches with consistent cargo sizes).
They’re able to cross the BBB, so they’re a very promising tool to deliver therapies to the brain for neurological disorders!
There are +100 clinical trials underway using exosomes as delivery vehicles, with a major focus on cancer and neurological diseases. Definitely a space to keep an eye on!
Coming up next…
In my next post I’ll share some of my learnings on: transposon systems for gene writing, RNA editing, CAR-T therapies, antisense oligonucleotides (ASOs)… I am mentioning them in case you’ve built an appetite and want to research them independently 🙂
Sources / to read (pending!! will be updated):
Prime editing: Nature article,, Wikipedia,, Addgene article
Topical gene edit: Nature article (with LNP: link)
Exosomes: Nature article
(Pending add from my Notion database)
